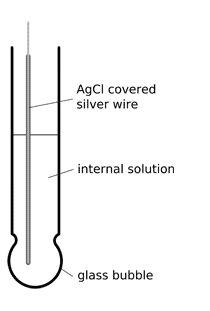
glass pH electrode
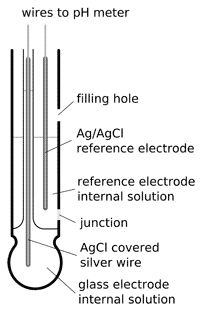
pH combination electrode
Most often used pH electrodes are glass electrodes. Typical model is made of a glass tube ending with a small glass bubble. Inside of the electrode is usually filled with buffered solution of chlorides in which silver wire covered with silver chloride is immersed. pH of internal solution varies - for example it can be 1.0 (0.1M HCl) or 7.0 (different buffers used by different producers).
Active part of the electrode is the glass bubble. While tube has strong and thick walls, bubble is made to be as thin as possible. Surface of the glass is protonated by both internal and external solution till equilibrium is achieved. Both sides of the glass are charged by the adsorbed protons, this charge is responsible for potential difference. This potential in turn is described by the Nernst equation and is directly proportional to the pH difference between solutions on both sides of the glass.
The majority of pH electrodes available commercially are combination electrodes that have both glass H+ ion sensitive electrode and additional reference electrode conveniently placed in one housing. For some specific applications separate pH electrodes and reference electrodes are still used - they allow higher precision needed sometimes for research purposes. In most cases combination electrodes are precise enough and much more convenient to use.
Construction of combination electrode is in large part defined by the processes that must take place when measuring pH. We need to measure difference of potentials between sides of glass in the glass electrode. To do so we need a closed circuit.
Circuit is closed through the solutions - internal and external - and the pH meter. However, for correct and stable results of measurements reference electrode must be isolated from the solution so that they will not crosscontaminate - and it is not an easy task to connect and isolate two solutions at the same time.
Connection is made through a small hole in the electrode body. This hole is blocked by porous membrane, or ceramic (asbestous in older models) wick. Internal solution flows very slowly through the junction, thus such electrodes are called flowing electrodes. To slow down the leaking, in gel electrodes internal solution is gelled.


