Ideal pH electrode should have potential dependent solely on the activity of the H+. Unfortunately, there is no such thing as ideal pH electrode.
Potential that builds up on the electrode surfaces has its source in the ions attaching themselves to the glass surface. Glass structure is such that only single charged ions are attracted. Depending on the ion this effect can be stronger or weaker, but the result will be always the same - other ions will interfere with the determination of pH.
To describe effect of other ions on the electrode potential we can use slightly simplified version of Nicolsky-Eisenman equation:
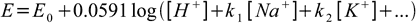
where ki are so called selectivity coefficients, determined experimentally.
Every glass electrode potential depends not only on pH but on concentrations of all other single charged ions present. Carefull selection of the glass composition is crucial, as glass is solely responsible for the selectivity coefficients values. These can take values from the 10-1 - 10-15 range. The smaller the value the better. Importance of the small selectivity coefficient can be shown with simple example. Let's assume selectivity coefficient H+/Na+ of 10-8 and 0.1M Na+ solution:
| real pH | measured pH |
|---|---|
| 1.00 | 1.00 |
| 7.00 | 7.00 |
| 8.00 | 7.96 |
| 9.00 | 8.70 |
| 10.00 | 8.96 |
Measurement will never show pH above 9.00 in this case. This effect is called alkaline error or sodium error, but not only sodium can interfere with pH measurements. Other single charged cations interfere as well. It is especially important in the case of buffers (for example TRIS based) where the concentration of interfering ion can be relatively high. Most commercial pH electrodes have selectivity ceofficients high enough to not allow such situations. Detailed information about selectivity should be available from electrode manufacturer.
It is worth of noting here, that using proper glass one can make glass electrode that can be used for determination of other single charged ions - like Ag+, Na+, K+ and so on.


